q10 temperature coefficient
This function can be used in two ways. The lab report Use Equation 1 to calculate Q10 using your own data from the lab.

Q 10 The Temperature Coefficient Youtube
Write the values of R1 R2 T1 and T2 on a piece of paper.
. If four of the first five parameters are given Q10 R1 R2 T1 T2 then the fifth parameter is returned or 2. The usual model for the Q 10 coefficient is expressed by the following equation. However this dependence is small for the temperature ranges within which ion channels operate.
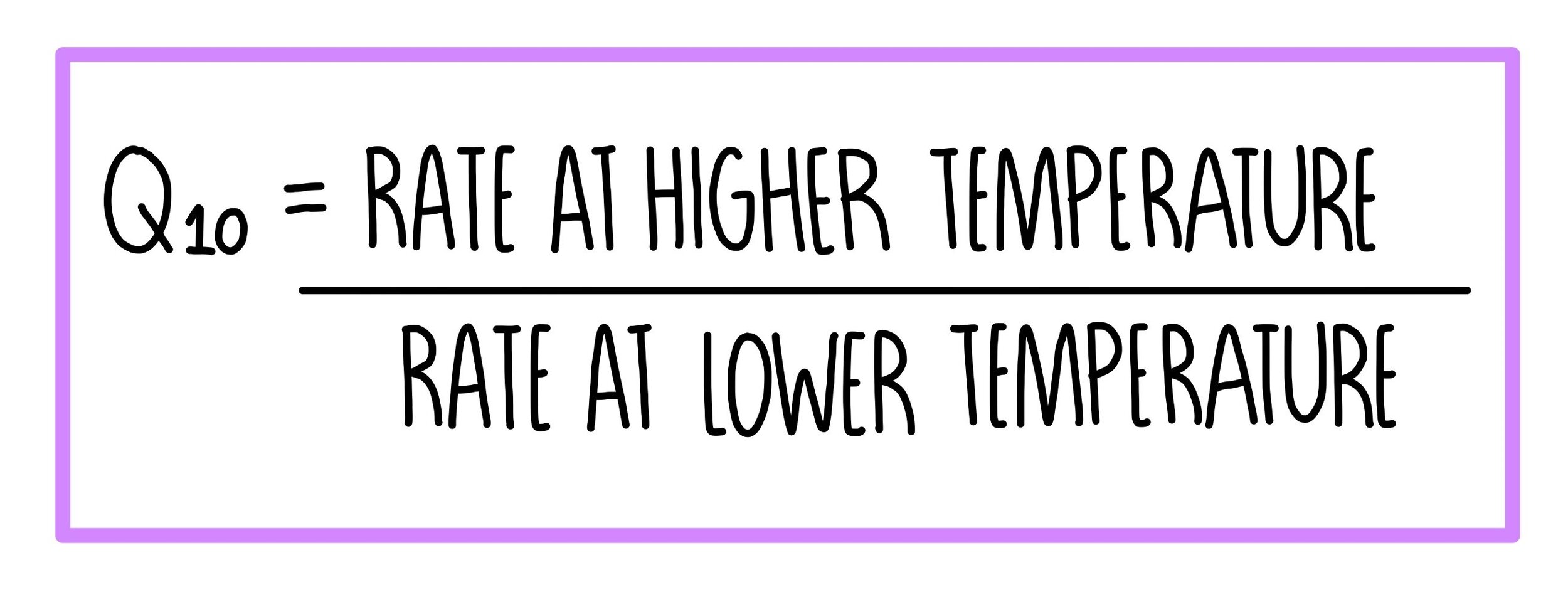
Q 10 10 C temperature driven chemical or biological process rate. It is defined as the ratio between the rate of a biological process at two temperatures separated by 10 C. Im not sure but your first equation has rate of Tc on the denominator whereas your equations just have Tc.
The Q10 coefficient represents the degree of temperature dependence a muscle exhibits as measured by contraction rates. This Q 10 coefficient is also used to measure the rate of change of chemical reactions. Calculates parameters from Q10 temperature coefficient for chemical or biological systems.
If R_vec and T_vec are given then the best Q10 for those data is returned. T2 Higher temperature t1 Lower temperature k2 Metabolic rate at t2 k1 Metabolic rate at t1 Q10 Coefficient Q10. The Q10 equation is then used to estimate the Q10 for the process.
The Q10 value is tied to an increase in the surrounding temperature with an increase in 10 C and usually resulted in a doubling of the reaction rate. Q10 temperature coefficient The RGT rule reaction rate - temperature control and van t Hoff rule is a rule of thumb in chemical kinetics and allows the estimation of many phenomena in chemistry biochemistry and ecology. Q10 temperature coefficient Quick Reference A measure of the effect of a 10 C rise in temperature on the velocity of a chemical reaction.
For example if R1 8 and R2 24 compute 248 3. The formula for Q10 coefficient is. When this happens the Q10 value for the.
Q 10 k T 2 k T 1 10 T 2 T 1 where T is the temperature in Celsius degrees or Kelvin and k is the rate constant expressed as exponential decay with temperature commonly expressed by the Arrhenius equation k A e E o R T where A E o and R are the frequency factor. This function can be used in two ways. The Q 10 is a measure of the degree to which a biological process depends on temperature.
R1 is the measured reaction rate at temperature T1 where T1. There are many examples where the Q10 is used one being the calculation of the nerve conduction velocity and another being calculating the contraction velocity of muscle fibres. Calculates parameters from Q10 temperature coefficient for chemical or biological systems.
Q10 is a unitless quantity. For example if the Q 10 of a reaction calculated from rates at 5 C and 15 C is found to be 3 the Q 10 calculated at 27 C and 37 C would be 257 a reduction of 14. There are many examples where the Q10 is used one being the calculation of the nerve conduction velocity and another being calculating the contraction velocity of muscle fibres.
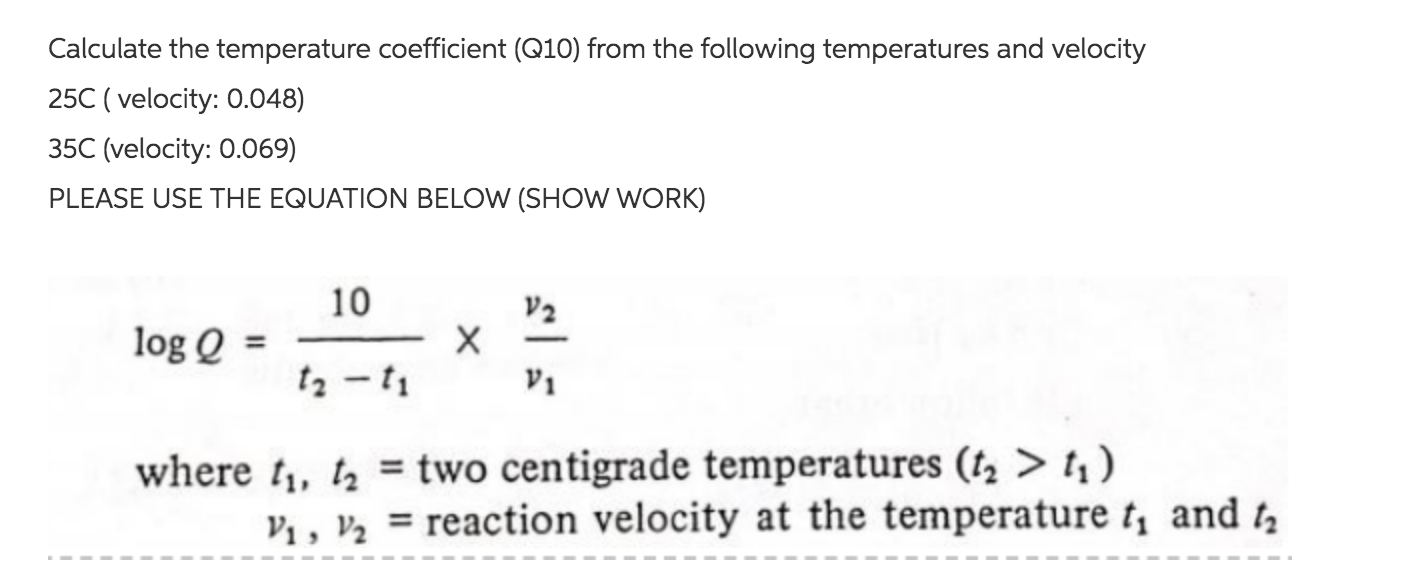
The temperature coefficient Q10 is calculated by measuring the rate of a reaction R at two different temperatures in Celsius degrees or kelvin and where T1. If R_vec and T_vec are given then the best Q10 for those data is returned. Compute R2R1 and write down your answer.
He then gives you an example. A COMMON method of comparing rates of reactions or processes in biological systems is the use of the temperature coefficient or Q10 the ratio of the rates of a reaction or process at T10 C. A Q10 of 10 indicates thermal independence of a muscle whereas an increasing Q10 value indicates increasing thermal dependence.
This yield an R1 at T1 and R2 at T2. This Q 10 coefficient is used to represent nerve conduction velocity and the contraction velocity of muscle fibers. The Q 10 is expressed as the ratio of the velocity of a chemical reaction at a given temperature to that of the same reaction at a temperature 10 C lower.
Q 10 k 2 k 1 10 t 2 t 1 Q 10 k 2 k 1 10 t 2-t 1. It states that chemical reactions at a 10 K increase in temperature take place two to four times as fast. Choose units and enter the following.
The Q10 temperature coefficient is the factor by which a rate of reaction such as a chemical reaction increases for each ten-degree increase in the temperature measured in degrees Celsius. If four of the first five parameters are given Q10 R1 R2 T1 T2 then the fifth parameter is returned or 2. Calculated by visually measuring growth rates k on glucose minimal medium at 37C and 23C according to equation Q10 k37k23 10 37-23 1038 10141996.
As can be seen in figure this value is constant in the temp. Temperature coefficient Q10 equation Q10 is the factor by which the reaction rate increases when the temperature is raised by ten degrees. The Q10 temperature coefficient is a measure of the rate of change of a biological or chemical system as a consequence of increasing the temperature by 10 C.
If you know the 1st equation is correct then putting in your solutions can check whether it is right. The Coefficient Q10 calculator computes the unitless measure of the rate of change of a biological or chemical process as a consequence of increasing the systems temperature by 10 C. 121M subscribers In this video Paul Andersen defines Q10 as the ratio between reactions at different temperatures.
In the context of ion channels it can be applied to the temperature dependence of the rate of channel opening and closing and to the dependence of. Thus the Q 10 is expected to depend on the measurement temperature. The Q10 temperature coefficient is a measure of the rate of change of a biological or chemical system as a consequence of increasing the temperature by 10 C.

Temperature Coefficient I E Q10 Of Heart Rate Dashed Line And Download Scientific Diagram

Q10 The Temperature Coefficient Youtube
Enzymes A Level The Science Hive
Solved Calculate The Temperature Coefficient Q10 From The Chegg Com
Temperature Coefficient I E Q10 Of Heart Rate Open Circles Dashed Download Scientific Diagram

Q10 Temperature Coefficient Wikiwand
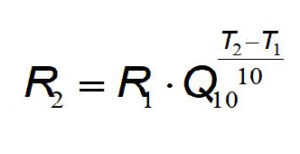
Effect Of Temperature On Oxygen Consumption Larvae Knowledge Incubator

Relationships Between The Q10 Temperature Coefficient For Respiration Download Scientific Diagram
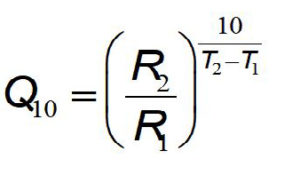
Effect Of Temperature On Oxygen Consumption Larvae Knowledge Incubator

Q10 Temperature Coefficient Ppt Download
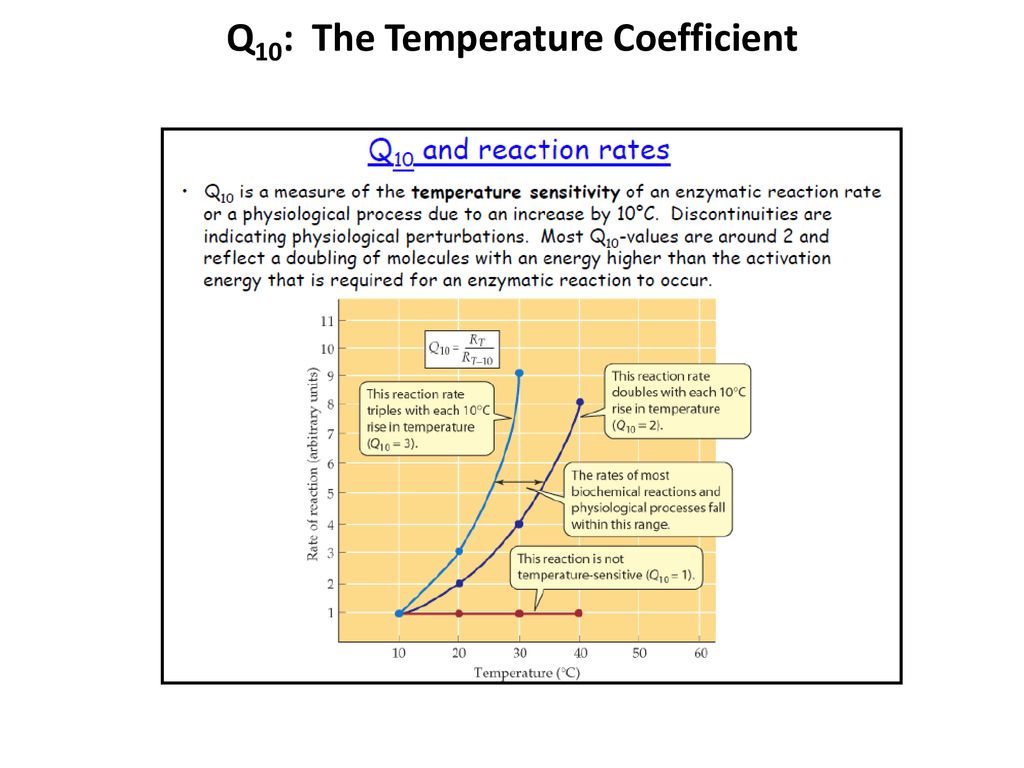
Thermoregulation And Q10 The Temperature Coefficient Ppt Download
Values Of The Temperature Coefficient Q10 During Hypothermia Calculated Download Scientific Diagram
Temperature Coefficient Q10 Calculations The Student Room

File Q10temperaturecoefficientplot Svg Wikipedia

Effect Of Temperature Coefficient Q10 And Acclimation Time On The Download Scientific Diagram
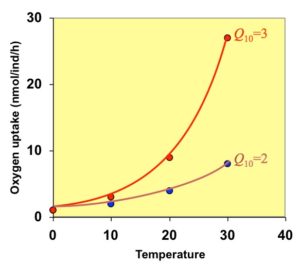
Effect Of Temperature On Oxygen Consumption Larvae Knowledge Incubator
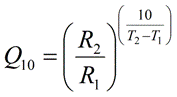
Temperature Coefficient Q10 Calculator Physiologyweb

Pdf Estimation Of The Q10 Value The Temperature Coefficient For The Growth Of Pseudomonas Sp Aq5 04 On Phenol

Mr I Explains How To Calculate The Temperature Coefficient Q10 For A Chemical Reaction Youtube



0 Response to "q10 temperature coefficient"
Post a Comment